
PD Dr. rer. nat. Susanne Sievers
Group Leader
Department of Microbial Physiology and Molecular Biology
Felix-Hausdorff-Straße 8 - room-nr.: 1.49
17489 Greifswald
phone: +49 (0)3834 420 5900
fax: +49 (0)3834 420 5909
email: sieverss(at)uni-greifswald(dot)de
| currently | head of Department of Microbial Physiology and Molecular Biology |
| 2024 | habilitation and venia legendi in microbiology and molecular biology |
| 2017 – 2021 | group leader in the Institute of Microbiology, University Greifswald |
| 2016 – 2017 | maternity leave |
| 2014 – 2016 | post-doc with Katharina Riedel, University Greifswald |
| 2013 – 2014 | maternity leave |
| 2011 – 2013 | postdoctoral fellowship of the German research foundation with Birgitte Kallipolitis at the SDU, Odense, Denmark |
| 2010 – 2011 | maternity leave |
| 2009 – 2010 | postdoc in the group of Michael Hecker, University Greifswald |
| 2005 – 2009 | dissertation on the „Establishment and application of non-2-D gel-based techniques for identification and quantification of challenging protein classes in Gram-positive bacteria” with Michael Hecker, University of Greifswald |
| 2003 – 2004 | visiting researcher in the group of Ursula Jakob, University of Michigan, Ann Arbor, USA |
| 09/2000 – 09/2005 | study of Biochemistry, University of Greifswald |
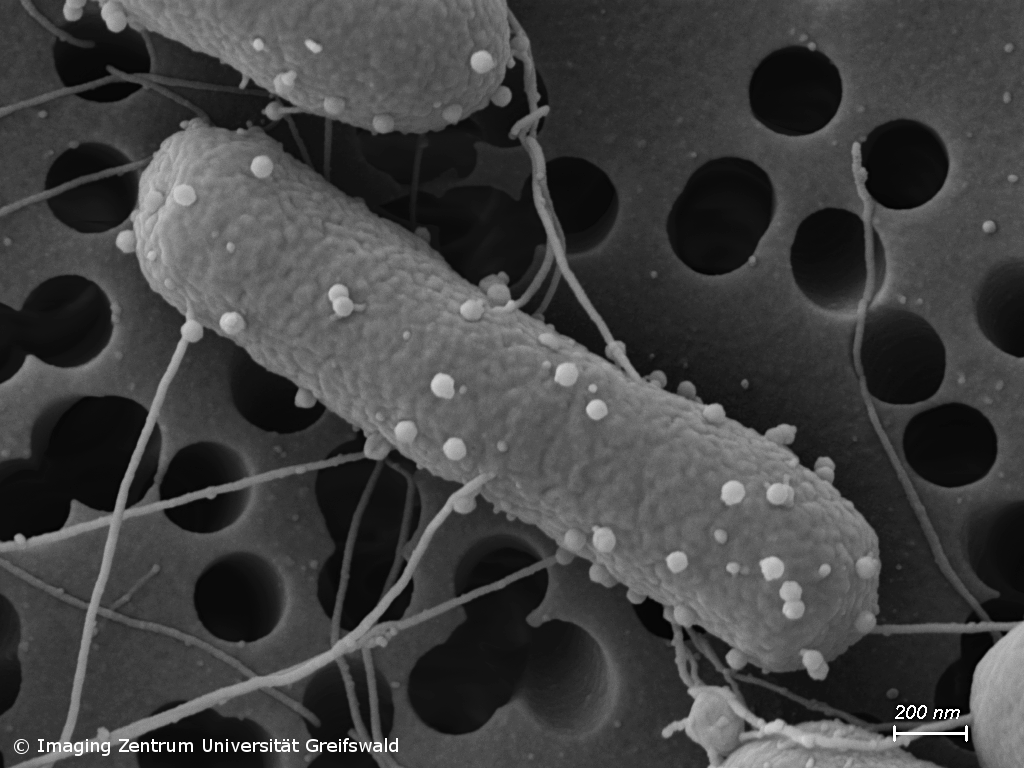
Antimicrobial resistance (AMR) and novel antimicrobial strategies
Infections with antimicrobial resistant pathogens are one of the major threats of mankind. Already today, clinicians have to deal with bacteria that show resistance to all currently available antibiotics. Our group studies occurrence and transmission of pathogens carrying AMR genes, e.g. in waste water and soil, but we also conduct research on the development of alternative antimicrobial therapies. For instance, we analyze the activity and mode of action of novel synthetic and natural substances against problematic bacterial pathogens, but also hunt for bacteriophages with the potential to be further developed to be perspectively used in phage therapy.
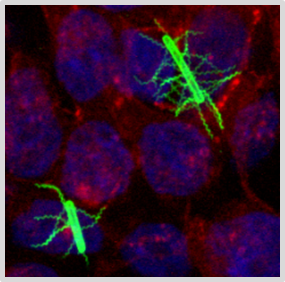
Pathophysiology of Clostridioides difficile (CDinfect)
The strictly anaerobic bacterium C. difficile is one of the most problematic pathogens causing nosocomial and relapsing infections. The intestinal pathogen can survive in the gut and establish infections due to its ability to form highly resistant endospores and to withstand the harsh conditions in the human intestinal tract. Despite the importance of C. difficile infections, the physiology of the pathogen is still not very well understood. We aim to pinpoint the main metabolic pathways and physiological efforts that empower C. difficile to thrive in the intestinal tract and to cause severe infections in humans.
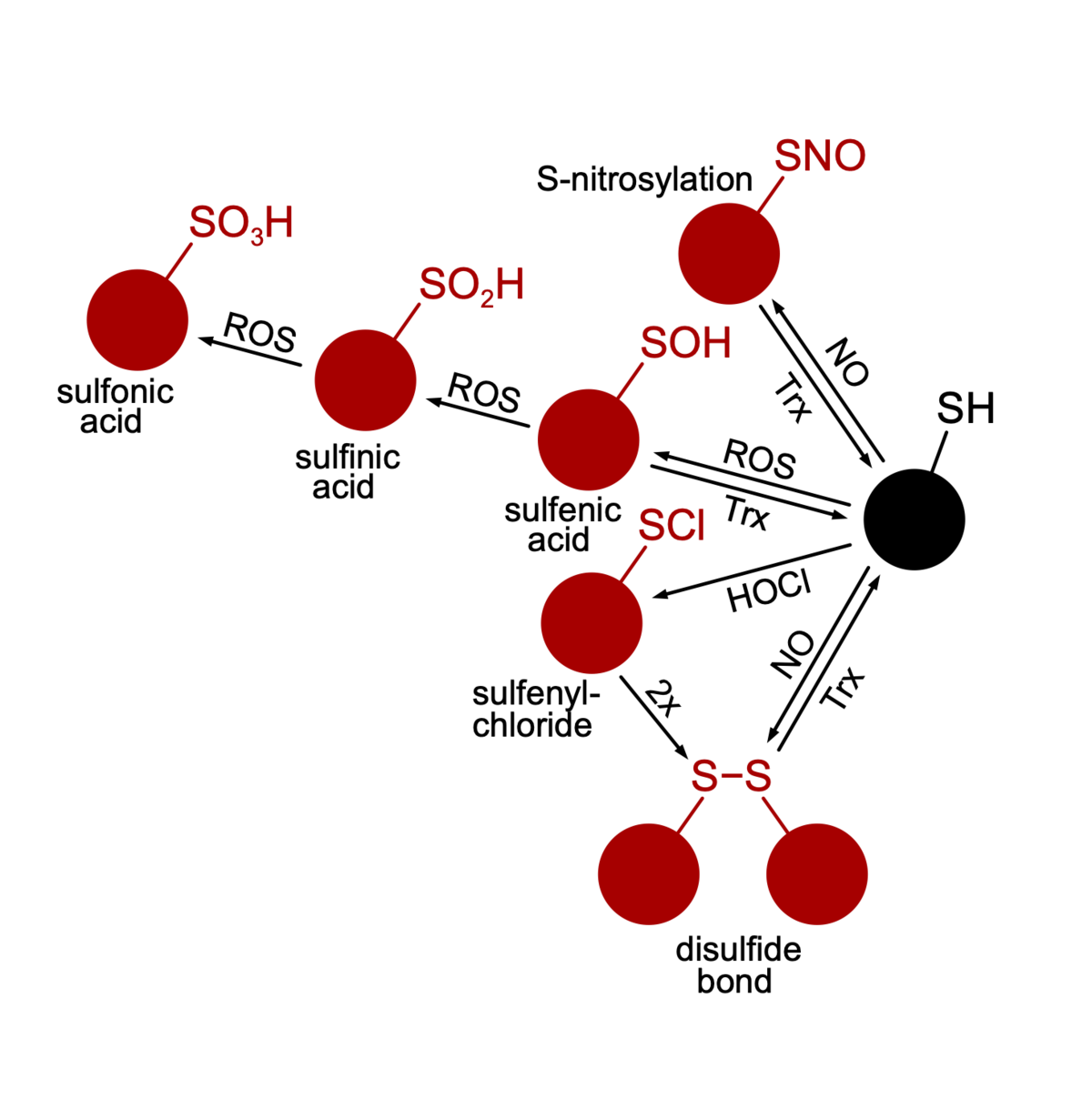
Oxidative stress and anti-oxidative strategies (BiOx GRK1947, FlavOx DFG)
Life is based on redox reactions, i. e. the cell is full of molecules that reduce or oxidize each other. Some molecules have a bigger oxidative potential than others. Especially in the presence of oxygen, reactive oxygen species (ROS) evolve that have the potential to oxidize cellular structures instigating signal transduction pathways or damaging macromolecules. Microorganisms that depend on oxygen in their metabolism feature sophisticated stress response-, repair- and ROS-detoxification mechanisms. One could assume that strictly anaerobic organisms are devoid of such mechanisms since they grow in habitats free of oxygen. However, many strictly anaerobic bacteria are capable of an oxidative stress response and can thus survive short periods of oxygen and ROS presence. With respect to anaerobic pathogens, the ability to survive periods of oxidative stress can decide on whether the pathogen can assert in the host or not. We study the oxidative stress response in C. difficile and thereby elucidate ROS sensors, signal transduction pathways, protective molecules and repair mechanisms. A special focus is put on oxidized proteins. Using mass spectrometry-based proteomics and redox-proteomics we aim to pinpoint redox switches and mostly vulnerable structures in the C. difficile cell.
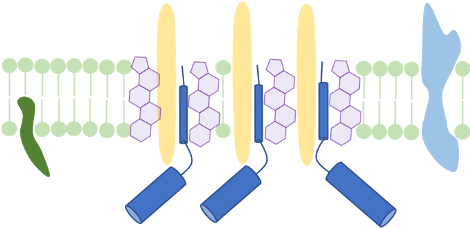
Membrane structure and membrane-active compounds
The cellular membrane separates the intracellular space from the exterior. It does not only accounts for the cell’s integrity, but also enables transport-, signal reception- and transduction processes. We make use of membrane proteomics and microscopic techniques to investigate the impact antimicrobial substances might have on membrane structure and function. Currently, we analyze changes of functional membrane microdomains in C. difficile in the presence of infection-relevant bile acids. We propose that host- or microbiome-derived membrane-active substances could have a combinatory effect with antimicrobial substances that are therapeutically used in infection.

Soil microbiome and its impact on agricultural crops (AgriBiom T!Raum One Health BMBF)
There is virtually no space on earth that is devoid of microbes. Specific niches feature their own specific microbial community – a niche-specific microbiome that plays an often still unknown role for the stability and equilibrium of this niche.Our expertise in mass-spectrometry-based proteomics and meta-proteomics enables us to perform in-depth analyses of the soil microbiome of agricultural grounds. On the one hand our aim is to unravel the microbes and their metabolic capabilities that are beneficial for the growth and health of agricultural crops and on the other hand identify microbial parameters that indicate deleterious effects emanated from the soil microbiome.
- Graf LG, Moreno-Yruela C, Qin C, …Susanne Sievers, et al. Distribution and diversity of classical deacylases in bacteria. Nat Commun. 2024;15(1):9496. PMID: 39489725
- Muñoz-Villagrán C, Acevedo-Arbunic J, Härtig E, et al. The thioredoxin fold protein (Tfp2) from extreme acidophilic leptospirillum sp. Cf-1 is a chaperedoxin-like protein that prevents the aggregation of proteins under oxidative stress. Int J Mol Sci. 2024;25(13):6905. PMID: 39000017
- Ferrara I, Chesnokov GA, Dittmann S, Blacque O, Sievers S, Gademann K. Formal single atom editing of the glycosylated natural product fidaxomicin improves acid stability and retains antibiotic activity. JACS Au. 2024;4(6):2267-2280. PMID: 38938792
- Kremer M, Schulze S, Eisenbruch N, Dirk Albrecht, Susanne Sievers et al. Bacteria employ lysine acetylation of transcriptional regulators to adapt gene expression to cellular metabolism. Nat Commun. 2024;15(1):1674. PMID: 38395951
- Troitzsch D, Knop R, Dittmann S, et al. Characterizing the flavodoxin landscape in Clostridioides difficile. Microbiol Spectr. Published online February 6, 2024:e0189523. PMID: 38319052
- Jung E, Kraimps A, Dittmann S, et al. Phenolic substitution in fidaxomicin: a semisynthetic approach to antibiotic activity across species. Chembiochem. 2023;24(24):e202300570. PMID: 37728121
- Bublitz A, Brauer M, Wagner S, et al. The natural product chlorotonil A preserves colonization resistance and prevents relapsing Clostridioides difficile infection. Cell Host Microbe. 2023;31(5):734-750.e8. PMID: 37098342
- Brauer M, Hotop SK, Wurster M, et al. Clostridioides difficile modifies its aromatic compound metabolism in response to amidochelocardin-induced membrane stress. mSphere. 2022 Sep-Oct; 7(5): e00302-22. PMID: 35993700
- Metzendorf NG, Lange LM, Lainer N, et al. Destination and specific impact of different bile acids in the intestinal pathogen clostridioides difficile. Front Microbiol. 2022;13:814692. PMID: 35401433
- Brauer M, Herrmann J, Zühlke D, Müller R, Riedel K, Sievers S. Myxopyronin B inhibits growth of a Fidaxomicin-resistant Clostridioides difficile isolate and interferes with toxin synthesis. Gut Pathog. 2022;14(1):4. PMID: 34991700
- Graf LG, Vogt R, Blasl AT, et al. Assays to study enzymatic and non-enzymatic protein lysine acetylation in vitro. Curr Protoc. 2021;1(11):e277. PMID: 34748287
- Brauer M, Lassek C, Hinze C, et al. What’s a biofilm? -how the choice of the biofilm model impacts the protein inventory of clostridioides difficile. Front Microbiol. 2021;12:682111. PMID: 34177868
- Troitzsch D, Zhang H, Dittmann S, et al. A point mutation in the transcriptional repressor perr results in a constitutive oxidative stress response in clostridioides difficile 630δerm. mSphere. 2021;6(2):e00091-21. PMID: 33658275
- Karyolaimos A, Dolata KM, Antelo-varela M, et al. Escherichia coli Can Adapt Its Protein Translocation Machinery for Enhanced Periplasmic Recombinant Protein Production. Front Bioeng Biotechnol. 2019;7:465. PMID:32064253
- Karyolaimos A, Ampah-korsah H, Hillenaar T, et al. Enhancing Recombinant Protein Yields in the Periplasm by Combining Signal Peptide and Production Rate Screening. Front Microbiol. 2019;10:1511. PMID:31396164
- Sievers S, Metzendorf NG, Dittmann S, et al. Differential View on the Bile Acid Stress Response of . Front Microbiol. 2019;10:258.PMID:30833939
- Guerrero montero I, Dolata KM, Schlüter R, et al. Comparative proteome analysis in an Escherichia coli CyDisCo strain identifies stress responses related to protein production, oxidative stress and accumulation of misfolded protein. Microb Cell Fact. 2019;18(1):19.PMID:30696436
- Dolata KM, Montero IG, Miller W, et al. Far-reaching cellular consequences of tat deletion in Escherichia coli revealed by comprehensive proteome analyses. Microbiol Res. 2019;218:97-107.
- Berges M, Michel AM, Lassek C, Nuss AM, Beckstette M, Dersch P, Riedel K, Sievers S, Becher D, Otto A, Maass S, Rohde M, Eckweiler D, Borrero-de Acuna JM, Jahn M, Neumann-Schaal M, Jahn D. Iron regulation in Clostridioides difficile. Front Microbiol. 2018. 9:3183. PMID:30619231
- Sievers S. Membrane Proteomics in Gram-Positive Bacteria: Two Complementary Approaches to Target the Hydrophobic Species of Proteins. Methods Mol Biol. 2018;1841:21-33.PMID:30259477
- Neumann-Schaal M, Metzendorf NG, Troitzsch D, Nuss AM, Hofmann JD, Beckstette M, Dersch P, Otto A, Sievers S. Tracking gene expression and oxidative damage of O-stressed Clostridioides difficile by a multi-omics approach. Anaerobe. 2018 May 31. pii: S1075-9964(18)30103-3 29859941.
- Sievers S, Dittmann S, Jordt T, Otto A, Hochgräfe F, Riedel K. Comprehensive redox profiling of the thiol proteome of Clostridium difficile. Mol Cell Proteomics.2018 Mar 1. PMID: 29496906.
- Otto A, Maaß S, Lassek C, Becher D, Hecker M, Riedel K, Sievers S. The protein inventory of Clostridium difficile grown in complex and minimal medium. Proteomics Clin Appl.2016 Oct;10(9-10):1068-1072. PMID: 27511832.
- Sievers S, Lund A, Menendez-Gil P, Nielsen A, Storm Mollerup M, Lambert Nielsen S, Buch Larsson P, Borch-Jensen J, Johansson J, Kallipolitis BH. The multicopy sRNA LhrC controls expression of the oligopeptide-binding protein OppA in Listeria monocytogenes. RNA Biol.2015 Jul 15:0. PMID: 26176322.
- Voigt B, Albrecht D, Sievers S, Becher D, Bongaerts J, Evers S, Schweder T, Maurer KH, Hecker M. High-resolution proteome maps of Bacillus licheniformis cells growing in minimal medium. Proteomics.2015 Apr 13. PMID: 25867794.
- Sievers S, Sternkopf Lillebæk EM, Jacobsen K, Lund A, Mollerup MS, Nielsen PK, Kallipolitis BH. A multicopy sRNA of Listeria monocytogenes regulates expression of the virulence adhesin LapB. Nucleic Acids Res.2014 Aug;42(14):9383-98. PMID: 25034691.
- Maass S, Sievers S, Zühlke D, Kuzinski J, Sappa PK, Muntel J, Hessling B, Bernhardt J, Sietmann R, Völker U, Hecker M, Becher D. Efficient, global-scale quantification of absolute protein amounts by integration of targeted mass spectrometry and two-dimensional gel-based proteomics. Anal Chem.2011 Apr 1;83(7):2677-84. PMID: 21395229.
- Sievers S, Ernst CM, Geiger T, Hecker M, Wolz C, Becher D, Peschel A. Changing the phospholipid composition of Staphylococcus aureus causes distinct changes in membrane proteome and membrane-sensory regulators. Proteomics.2010 Apr;10(8):1685-93. PMID: 20162562.
- Hempel K, Pané-Farré J, Otto A, Sievers S, Hecker M, Becher D. Quantitative cell surface proteome profiling for SigB-dependent protein expression in the human pathogen Staphylococcus aureus via biotinylation approach. J Proteome Res.2010 Mar 5;9(3):1579-90. PMID: 20108986.
- Becher D, Hempel K, Sievers S, Zühlke D, Pané-Farré J, Otto A, Fuchs S, Albrecht D, Bernhardt J, Engelmann S, Völker U, van Dijl JM, Hecker M. A proteomic view of an important human pathogen–towards the quantification of the entire Staphylococcus aureus proteome. PLoS One.2009 Dec 4;4(12):e8176. PMID: 19997597.
- Hahne H, Wolff S, Hecker M, Becher D. From complementarity to comprehensiveness–targeting the membrane proteome of growing Bacillus subtilis by divergent approaches. Proteomics.2008 Oct;8(19):4123-36. PMID: 18763711.
- Wolff S, Hahne H, Hecker M, Becher D. Complementary analysis of the vegetative membrane proteome of the human pathogen Staphylococcus aureus. Mol Cell Proteomics.2008 Aug;7(8):1460-8. PMID: 18460691.
- Wolff S, Antelmann H, Albrecht D, Becher D, Bernhardt J, Bron S, Büttner K, van Dijl JM, Eymann C, Otto A, Tam le T, Hecker M. Towards the entire proteome of the model bacterium Bacillus subtilis by gel-based and gel-free approaches. J Chromatogr B Analyt Technol Biomed Life Sci.2007 Apr 15;849(1-2):129-40. PMID: 17055787.
- Wolff S, Otto A, Albrecht D, Zeng JS, Büttner K, Glückmann M, Hecker M, Becher D. Gel-free and gel-based proteomics in Bacillus subtilis: a comparative study. Mol Cell Proteomics.2006 Jul;5(7):1183-92. PMID: 16552027.
- Kohler C, Wolff S, Albrecht D, Fuchs S, Becher D, Büttner K, Engelmann S, Hecker M. Proteome analyses of Staphylococcus aureus in growing and non-growing cells: a physiological approach. Int J Med Microbiol.2005 Dec;295(8):547-65. PMID: 16325551.
